how to find molar mass
When you are at the lab, you need to work with many different solutions. To create a solution with the correct concentration, you will need to be aware of how much of a given compound you're adding.
Say you have to earn a solution of 1M NaCl to get a reaction you're in charge of. How would you know how much salt to take out then add to a given quantity of water?
To do it, you will need to discover a means to relate the mass of a chemical (salt) to the amount of moles of the chemical. The molar mass of elements tells you the molar mass of a compound, so it's possible to figure out this.

Definition
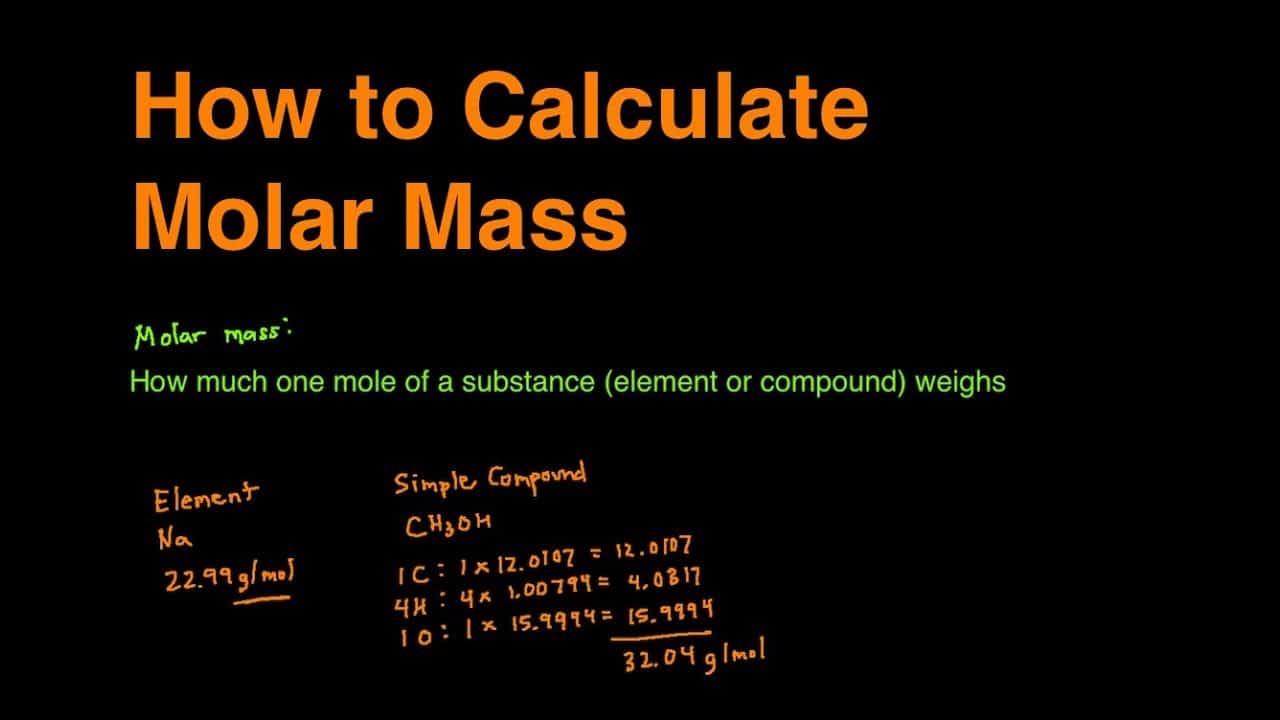
The molar mass definition is the grams of a material that are found in one mole of a chemical.
What is a mole of a substance? A mole of a material is a specific quantity of that material.
A mole is a number: 6.022 x 1023. Since particles of a compound are so modest, you can not really speak about them separately without it getting very cumbersome. Because of this, scientists make use of the mole. It describes that a huge number of molecules. Grams per mole hence clarifies the mass of a component whenever there are one-mole particles of the substance.
Now that you know what a mole is and exactly what molar mass refers to, where would you find this number? It is possible to locate it for every element on the periodic table. If you look under the symbol for each element, you will find a number. The molar mass of components is provided by that amount, molar mass, which is also the molecular weight. Related – How Much Does a Gallon of Water Weigh?
Molar Mass Cases
The amount under oxygen is 15.999 (have a look at a regular table on the internet or in your textbooks).
What about the molar mass of a chemical, not just an element?
To find the molar mass of a chemical, you have to bring the molar mass of all of the components in that chemical.
Have a look at NaOH which contains sodium, oxygen, and hydrogen. The molar mass of sodium is 22.99 g/mol, oxygen is 15.999 g/mol, and hydrogen is 1.008 g/mol.
Adding them together yields:
Imagine if you have a compound that has an element with a subscript? For example, MgCl2?
In this case, you would need to include the molar mass of magnesium and twice the molar mass of chlorine:
Using Molar Mass
Now, you can handle the question at the start of the report. Say you have to make 1 L of a 1M NaCl solution. How do you know how much salt to fat out?
First, you'll need to find the amount of moles you require for this solution. To do this, you will have to create use of the desirable molarity and the volume of the desired solution.
This calculation means you need a single spoonful of salt. Now, you need to use the molar mass of salt to calculate how much you will need to weigh out.
This calculation means that if you wished to create a 1-liter solution of 1M NaCl, you would have to weigh out 58.44 grams of NaCl. You would add this 58.44 grams of salt into a graduated cylinder and fill this up until the 1-liter mark.
More For You:
What's Concentration gradient?
how to find molar mass
Source: https://geteducationcrunch.com/how-to-find-molar-mass/
Posted by: davisanney1978.blogspot.com

0 Response to "how to find molar mass"
Post a Comment